Executive Summary
12/09/05
Adding severity adjustment measures to the HCUP Nationwide Inpatient Sample (NIS) and Kids’ Inpatient Database (KID) will significantly enhance the datasets for research purposes. An evaluation of alternative severity adjustment systems was conducted, focusing on those systems that require only hospital administrative data. The evaluation was conducted for AHRQ by an outside researcher who is not affiliated with any severity software vendor (Mark Hornbrook, Ph.D, Center for Health Research, Kaiser Permanente, Northwest Region). The goal of the evaluation was to select the best, most comprehensive measures of disease severity for inclusion in HCUP data. Four systems were selected to disseminate with the NIS and KID: APS-DRGs, APR-DRGs, Disease Staging, and the AHRQ comorbidity measures.
All-Payer Severity-adjusted (APS) DRGs (HSS, Inc.) and All-Patient Refined (APR) DRGs (3M) were selected as two alternative DRG-based systems to support research on topics such as design of improved hospital payment systems, risk-adjusted medical outcome studies, and measurement of hospital casemix. APR-DRGs and APS-DRGs are the culmination of separate lines of development in DRG-based severity adjustment and are built upon prior systems such as R-DRGs and AP-DRGs. They both include substantial refinements in severity specification and neonatal categories and they differ from one another in their trajectory of development, clinical logic, severity classification structure, and level of complexity.
Two clinically based measures of severity were also selected. Disease Staging (Medstat) was selected as a measure of disease severity that is not confounded by use of surgical procedures, as are DRG-based systems. Disease Staging also provides mortality, length of stay and total charge prediction scales that are equivalent to the DRG-based systems. The AHRQ comorbidity measures were selected to provide a measure of comorbidity burden beyond the principal diagnosis and its severity.
Basic documentation for all systems is distributed with the data. For the three proprietary systems (Disease Staging, APR-DRGs, and APS-DRGs), the NIS and KID documentation CD-ROM includes abbreviated documentation outlining the measures and their recommended use. Detailed documentation will also be available from the vendors for the proprietary systems at a special reduced price ($250-$400 depending on the vendor). This documentation will provide NIS and KID users with the complete clinical and coding logic that will describe the specific assignment of cases to individual disease categories/DRGs and levels of severity. Contact information for each vendor is provided at the end of this document. Documentation for the AHRQ comorbidity measures is available on the HCUP User Support Website (hcup-us.ahrq.gov).
Background and Rationale
Hospital administrative data have been widely used for examining issues related to payment, cost, utilization, and patient outcomes. All these topics require adjustment for patient severity of illness. Researchers can develop their own methods or select one of the severity measurement systems available in the public or private domain. With increased use of the Nationwide Inpatient Sample (NIS) and Kids’ Inpatient Database (KID) for various research purposes, adding severity measures will significantly enhance the research value of this database. An evaluation of existing severity measurement systems that are applicable to inpatient discharge data was conducted for AHRQ by an outside researcher independent of any severity software vendor.1 This document provides a summary of the evaluation and brief information about each selected system.
The purpose of the severity adjustment evaluation was to identify those severity measurement systems that are most applicable to the HCUP inpatient databases. There was no intention to rank specific products, but rather to aid in the selection of multiple products that will serve different purposes.
Results of the Evaluation
The evaluation results suggested that in addition to Medicare DRGs and AHRQ Clinical Classification System (CCS) which are already disseminated with HCUP data, severity measures from four systems should be added to the database -- Disease Staging,2 the AHRQ comorbidity measures,3 All Patient Refined (APR) DRGs,4 and All Payer Severity-adjusted (APS) DRGs.5 These systems are sufficiently differentiated in their technical properties making them unlikely substitutes for one another in any potential application (see Table 1). Each system has a relative advantage over the others depending on the purpose of the analysis.
Disease Staging
Disease Staging and the AHRQ comorbidity measures represent clinically based systems. Disease Staging is a product of Medstat. In Disease Staging, severity is defined as the likelihood of death or organ failure resulting from disease progression and independent of the treatment process.2 Disease progression is measured using four stages of increasing complexity and substages within Stages 1, 2, and 3. Stages and substages are defined specific to each disease category following clinical criteria:
Disease Staging uses information on diagnoses, sex, Cesarean section procedure codes, and discharge status to assign cases to disease categories (version 4 of the software includes 606 categories) and to measure disease-specific severity. Major operating room procedures are not used in assigning disease categories, thus use of procedures does not figure into the assignment of severity (as with DRG-based systems). However, to improve the value of Disease Staging in explaining costs, length of stay, and mortality, Medstat developed expense, stay and mortality predictive scales based on Medicare DRGs, using the DRG medical/surgical splits. Patient age, sex, and admission and discharge status are also used in these models. The following measures are included in the NIS and KID, based on Disease Staging software: disease categories and stages for the principal diagnosis and predictive scales for mortality, length of stay, and total charges.
AHRQ Comorbidity Measures
The AHRQ comorbidity measures identify coexisting medical conditions that are not directly related to the principal diagnosis, or the main reason for admission, and are likely to have originated prior to the hospital stay.3 These comorbidities can make a hospital stay more expensive and complicated. The AHRQ comorbidity measures were developed originally as one of the HCUP tools. Complete documentation on the comorbidity measures is available on the HCUP User Support Website under Tools & Software (https://hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp).
APR-DRGs and APS-DRGs
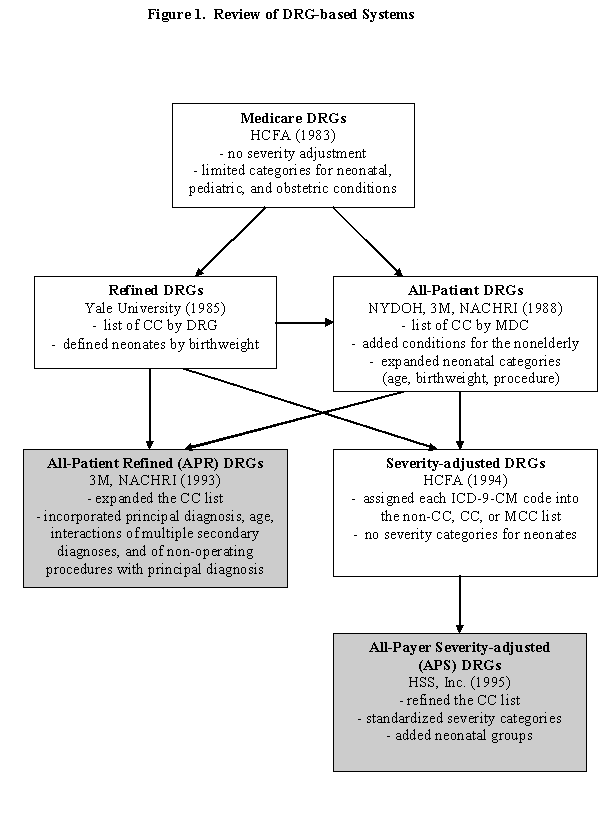
APR-DRGs and APS-DRGs are DRG-based severity measurement systems. A brief review of different DRG classification systems helps shed light on the rationale for selecting these two particular systems. DRG-based systems, as illustrated in Figure 1, include:
There are two major limitations in using Medicare DRGs for severity adjustment. First, there is limited adjustment for severity of illness.6 Principal diagnoses and procedures are stratified into categories based on the presence of a substantial complication or comorbidity (CC) in secondary diagnoses. The CC list includes about 3,000 diagnosis codes for diverse conditions that range from major acute illness to less severe chronic conditions. As a result, DRG categories are unable to sufficiently account for the differential effects of these secondary diagnoses on resource use. Second, Medicare DRGs have limited categories for conditions specific to the nonelderly, particularly for neonates,7 for whom there are only seven broadly-defined DRGs.
Developed during the mid to late 1980s, Refined DRGs (R-DRGs) and All-Patient DRGs (AP-DRGs) represented the first modifications of Medicare DRGs that attempted to account for severity of illness. Both systems address the limitations of DRGs through refinement of the CC list and the DRG categories for neonates.
With R-DRGs, developed by researchers at Yale University, the CC list for secondary diagnoses was specified separately for each DRG and the DRGs for neonates were revised to incorporate birthweight.6 AP-DRGs, first implemented in New York State, expanded Medicare DRGs to include neonatal, obstetric, and other conditions typical to the under-65 population.4 Based on research conducted by the National Association of Children’s Hospitals and Related Institutions (NACHRI), neonates were stratified by age, birthweight, and procedure.7 The assignment of CCs for secondary diagnosis was determined at the MDC level, rather than the DRG level.
AP-DRGs formed the basis for All-Patient Refined DRG (APR-DRGs) which were developed by 3M Health Information Systems in the early 1990s.4 APR-DRGs added severity of illness and risk of mortality subclasses for each base APR-DRG. In determining the severity level, 3M not only revised the CC list to accommodate the non-Medicare population but also incorporated principal diagnosis, age, interactions of multiple secondary diagnoses, and combinations of non-operating procedures with principal diagnosis. The severity of illness and risk of mortality subclasses have levels of 1 to 4, indicating minor, moderate, major, and extreme, respectively. Based on these enhancements, APR-DRGs represented a significant improvement over both R-DRGs and AP-DRGs.
In 1994, the Center for Medicare and Medicaid Services (CMS, formerly HCFA) developed Severity-adjusted DRGs (S-DRGs) which incorporated aspects of R-DRGs and AP-DRGs.8 The major enhancement of S-DRGs lies in the revision of the CC list. Instead of defining the CC list by DRG (like the R-DRG system) or by MDC (like the AP-DRG system), researchers at CMS evaluated the presence of each ICD-9-CM diagnosis code as a secondary diagnosis to determine its effect on resource use. Based on this analysis, each code was assigned as a major CC (MCC), CC, or non-CC. Two concerns with S-DRGs are: 1) no refinement for neonatal groups, making this system inapplicable to an all-payer population; and 2) inconsistent severity categories across DRGs.
To address these limitations, HSS Inc. introduced All-Payer Severity-adjusted DRG (APS-DRGs) in 1995.9 As an expansion of S-DRGs, APS-DRGs are intended to be used for all hospitalized patients. Neonatal categories are defined by birthweight, diagnoses, and disposition. APS-DRGs also extend the exclusion criteria inherited from Medicare DRGs and S-DRGs to eliminate certain secondary diagnoses from the CC and MCC lists because those conditions are associated with the principal diagnosis. The severity categories are standardized across DRGs with 0, 1, and 2 denoting without CC, with CC, and with MCC, respectively.
In summary, APR-DRGs and APS-DRGs are built upon prior DRG-based systems with substantial refinements in severity specification and neonatal categories. These two systems differ from one another in their trajectory of development, clinical logic, severity classification structure, and level of complexity.
APR-DRGs (version 20) include 316 base disease categories. The following measures are included for APR-DRGs: base APR-DRG, severity of illness subclass, and risk of mortality subclass within each base APR-DRG.
APS-DRGs (version 20) consist of 375 base disease categories called Consolidated DRGs (CDRGs). The APS-DRG group number is represented by the CDRG category (XXX) plus the one-digit severity class (Y). Weights developed using the NIS for mortality, length of stay, and total charges are also included.
Documentation
Documentation for all systems is distributed with the NIS. For the three proprietary systems (Disease Staging, APR-DRGs, and APS-DRGs) abbreviated documents outlining the measures and their recommended use are included with the NIS. For users who wish to understand the specific assignment of cases to individual disease categories/DRGs and levels of severity, full documentation that provides the complete clinical and coding logic will be available from the vendors at a special reduced price. Contact information for each product is provided below.
| For detailed documentation on APR-DRGs, please contact: | Cheryl Rothermich, RN Product Marketing Manager All Patient Refined DRGs (APR-DRGs) 3M Health Information Systems clrothermich@mmm.com 801-265-4427 |
| For detailed documentation on APS-DRGs, please contact: | HSS, Inc. 2321 Whitney Ave. 4th Fl. Hamden, CT 06518 APS-DRGs@hss-info.com |
| For detailed documentation on Disease Staging, please contact: | Scott McCracken Product Manager Medstat 777 E Eisenhower Pkwy Ann Arbor, MI 48169 scott.mccracken@thomson.com |
| For detailed documentation on the AHRQ comorbidity meaures please contact: | HCUP User Support Website hcup-us.ahrq.gov |
References
| Name of System | Source | Definition of Severity | Input Variables | Output Variables |
|---|---|---|---|---|
| Disease Staging, version 5.21.3 | Medstat | Likelihood of death or organ failure as a result of disease progression, independent of treatment |
- Diagnoses - Discharge status - Sex - Procedures |
- Disease category - Stage 1.x, 2.x, 3.x, 4.0 with substages available for stages 1, 2, and 3; number of subscales varies across diseases; stage 4 denotes death - Predictive scales for mortality, length of stay, and total charges (recalibrated for the NIS) |
| AHRQ Comorbidity Measures, version 2.0 | AHRQ | Coexisting medical conditions that are not directly related to the principal diagnosis, or the main reason for admission, and are likely to have originated prior to the hospital stay. |
- Diagnoses - DRGs |
30 dummy variables (1, 0) indicating the presence of each comorbid condition |
| All-Payer Severity-adjusted DRG (APS-DRGs), version dependent on discharge date | HSS | Resource intensity in terms of lengthy stay or high charges/cost. Version 19.0 for discharges before 10/1/2002. Version 20.0 for discharges from 10/1/2002 to 9/30/2003. Version 21.0 for discharges after 9/30/2003. |
- Diagnoses - Procedures - Discharge Status - Age - Length of Stay - Birthweight (if present) |
- APS-DRG category: XXXY, where XXX is the Consolidated DRG and Y is the severity class (0, 1, 2); severity level 0 to 2 indicates no CC or major CC, with CC, and with major CC, respectively; CC stands for complications or comorbidities - Weights for mortality, length of stay, and total charges |
| Internet Citation: Overview of Disease Severity Measures. Healthcare Cost and Utilization Project (HCUP). September 2025. Agency for Healthcare Research and Quality, Rockville, MD. hcup-us.ahrq.gov/db/nation/nis/severity_overview.jsp. |
| Are you having problems viewing or printing pages on this website? |
| If you have comments, suggestions, and/or questions, please contact hcup@ahrq.gov. |
| If you are experiencing issues related to Section 508 accessibility of information on this website, please contact hcup@ahrq.gov. |
| Privacy Notice, Viewers & Players |
| Last modified 9/29/25 |